Akuaporin 1
Akuaporin 1 adalah protein pada manusia yang dikodekan oleh gen AQP1.
AQP1 secara luas mengekspresikan saluran air yang fungsi fisiologisnya banyak dikarakteristikkan di dalam ginjal. Protein ini ditemukan pada membran plasma basolateral dan apikal tubulus proksimal, lengan menurun lengkung Henle, dan pada bagian menurun vasa rekta. Sebagai tambahan, protein ini juga ditemukan pada sel darah merah, endotelium vaskular, saluran pencernaan, kelenjar keringat, dan paru-paru.
Akuaporin ini tidak diregulasi oleh vasopresin (ADH).
Fungsi
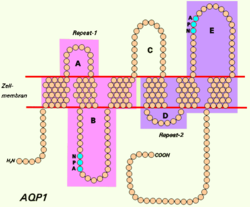
suntingAkuaporin merupakan famili protein membran integral kecil terkait protein intrinsik mayor (MIP atau AQP0). Gen ini mengodekan akuaporin yang berfungsi sebagai protein saluran air molekuler. Protein ini merupakan homotetramer dengan 6 domain rentangan dwilapis dan situs N-glikosilasi. Fisik protein menyerupai protein saluran dan melimpah pada eritrosit dan tubulus ginjal. Gen yang mengodekan akuaporin ini menjadi kandidat mungkin untuk kelainan yang melibatkan ketidakseimbangan pada pergerakan cairan okular.[4]
Lihat juga
suntingReferensi
sunting- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000004655 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: AQP1 aquaporin 1 (Colton blood group)".
Bacaan lanjutan
sunting- Knepper MA (1994). "The aquaporin family of molecular water channels". Proc. Natl. Acad. Sci. U.S.A. 91 (14): 6255–8. doi:10.1073/pnas.91.14.6255. PMC 44179 . PMID 7517546.
- Borgnia M, Nielsen S, Engel A, Agre P (2000). "Cellular and molecular biology of the aquaporin water channels". Annu. Rev. Biochem. 68: 425–58. doi:10.1146/annurev.biochem.68.1.425. PMID 10872456.
- Horster M (2001). "Embryonic epithelial membrane transporters". Am. J. Physiol. Renal Physiol. 279 (6): F982–96. PMID 11097616.
- Yool AJ, Weinstein AM (2002). "New roles for old holes: ion channel function in aquaporin-1". News Physiol. Sci. 17: 68–72. PMID 11909995.
- Mitra AK, Ren G, Reddy VS, Cheng A, Froger A (2002). "The architecture of a water-selective pore in the lipid bilayer visualized by electron crystallography in vitreous ice". Novartis Found. Symp. Novartis Foundation Symposia. 245: 33–46; discussion 46–50; 165–8. doi:10.1002/0470868759.ch4. ISBN 978-0-470-86875-1. PMID 12027013.
- Ripoche P, Goossens D, Devuyst O, Gane P, Colin Y, Verkman AS, Cartron JP (2006). "Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: a stopped-flow analysis". Transfusion clinique et biologique : journal de la Société française de transfusion sanguine. 13 (1–2): 117–22. doi:10.1016/j.tracli.2006.03.004. PMID 16574458.
- Preston GM, Carroll TP, Guggino WB, Agre P (1992). "Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein". Science. 256 (5055): 385–7. doi:10.1126/science.256.5055.385. PMID 1373524.
- Preston GM, Agre P (1992). "Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family". Proc. Natl. Acad. Sci. U.S.A. 88 (24): 11110–4. doi:10.1073/pnas.88.24.11110. PMC 53083 . PMID 1722319.
- Smith BL, Agre P (1991). "Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins". J. Biol. Chem. 266 (10): 6407–15. PMID 2007592.
- Denker BM, Smith BL, Kuhajda FP, Agre P (1988). "Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules". J. Biol. Chem. 263 (30): 15634–42. PMID 3049610.
- Zelinski T, Kaita H, Lewis M, Coghlan G, Philipps S, Belcher E, McAlpine PJ, Coopland G, Wong P (1988). "The Colton blood group locus. A linkage analysis". Transfusion. 28 (5): 435–8. doi:10.1046/j.1537-2995.1988.28588337331.x. PMID 3166547.
- Preston GM, Jung JS, Guggino WB, Agre P (1994). "Membrane topology of aquaporin CHIP. Analysis of functional epitope-scanning mutants by vectorial proteolysis". J. Biol. Chem. 269 (3): 1668–73. PMID 7507481.
- Skach WR, Shi LB, Calayag MC, Frigeri A, Lingappa VR, Verkman AS (1994). "Biogenesis and transmembrane topology of the CHIP28 water channel at the endoplasmic reticulum". J. Cell Biol. 125 (4): 803–15. doi:10.1083/jcb.125.4.803. PMC 2120064 . PMID 7514605.
- Li X, Yu H, Koide SS (1994). "The water channel gene in human uterus". Biochem. Mol. Biol. Int. 32 (2): 371–7. PMID 7517253.
- Walz T, Smith BL, Agre P, Engel A (1994). "The three-dimensional structure of human erythrocyte aquaporin CHIP". EMBO J. 13 (13): 2985–93. PMC 395186 . PMID 7518771.
- Preston GM, Smith BL, Zeidel ML, Moulds JJ, Agre P (1994). "Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels". Science. 265 (5178): 1585–7. doi:10.1126/science.7521540. PMID 7521540.
- Smith BL, Preston GM, Spring FA, Anstee DJ, Agre P (1994). "Human red cell aquaporin CHIP. I. Molecular characterization of ABH and Colton blood group antigens". J. Clin. Invest. 94 (3): 1043–9. doi:10.1172/JCI117418. PMC 295159 . PMID 7521882.
- van Hoek AN, Wiener MC, Verbavatz JM, Brown D, Lipniunas PH, Townsend RR, Verkman AS (1995). "Purification and structure-function analysis of native, PNGase F-treated, and endo-beta-galactosidase-treated CHIP28 water channels". Biochemistry. 34 (7): 2212–9. doi:10.1021/bi00007a015. PMID 7532004.
- Keen TJ, Inglehearn CF, Patel RJ, Green ED, Peluso DC, Bhattacharya SS (1995). "Localization of the aquaporin 1 (AQP1) gene within a YAC contig containing the polymorphic markers D7S632 and D7S526". Genomics. 25 (2): 599–600. doi:10.1016/0888-7543(95)80070-3. PMID 7540589.
Pranala luar
sunting- MeSH Aquaporin+1
- Galeri Simulasi Akuaporin
- Lokasi genom AQP1 manusia dan halaman rincian gen AQP1 dalam UCSC Genome Browser.
Artikel ini menggabungkan teks dari Perpustakaan Kedokteran Nasional Amerika Serikat yang berada dalam domain publik.